We are exploring the large-area integration of van der Waals solids and other nanomaterials using top-down solution-based chemical exfoliation. Here chemicals and mechanical agitation can be used to shear the layers from the bulk crystals, to yield inks that can be used in an additive manufacturing platform for enabling devices using scalable approaches over a wide range of substrates.

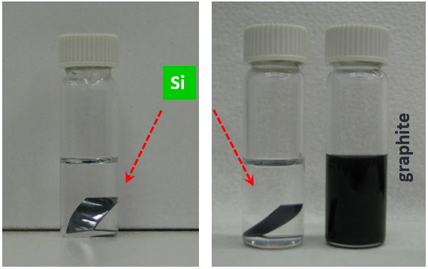
Bulk graphite, a well-explored van der Waals solid, is shown on the left which yields a dark ink (right-most vial) when placed in a conventional organic solvent (NMP), while Si, a 3D non van der Waals crystal, remains in-tact (left vials).
We utilize techniques such as Optical Absorption Spectroscopy, Raman Spectroscopy and Atomic Force Microscopy to characterize the structural and chemical properties of the materials within the dispersions.
Excitonic Effects in Solution Dispersions of MoS2 and WS2
Recently we have explored excitonic effects which are elucidated for solution dispersions of MoS2 and WS2. The excitonic enhancement effects are correlated to new solution chemistries. The role of sonication time and centrifugation are analyzed in the presence of terpineol with isopropyl alcohol and surfactant ethyl cellulose, where the red-shift in the excitonic peaks is correlated to the size distribution of the nanoparticles using optical interferometry. This was correlated to the data obtained using photoluminescence, Raman spectroscopy, scanning electron microscopy and particle size analysis. Analysis of the optical absorption spectra allows for the extraction of the energy band gap for MoS2 and WS2. These results clearly show evidence of quantum confinement effects in solution dispersions of chemically exfoliated 2D MoS2 and WS2 which can be harnessed for a wide variety of optoelectronic devices. We have also shown the solution chemistries that enhance excitonic effects also lead to devices such as p-n junctions that also lead to lower leakage currents in the reverse bias regime and lower turn-on voltages. For more details on this research please click here. This research was featured as a back cover image in the Journal of Materials Chemistry C.
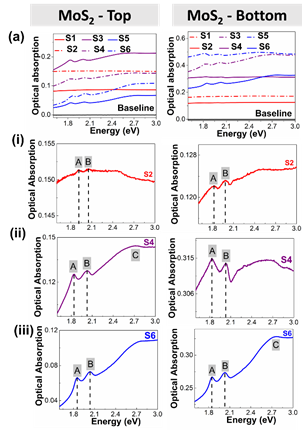
Optical absorption spectra taken from top and bottom of vial for (a) MoS2 taken from (left) the top of the vial, and (right) bottom of the vial. Similar analysis was conducted for WS2. The peaks shown are the A, B and C excitonic peaks for MoS2.
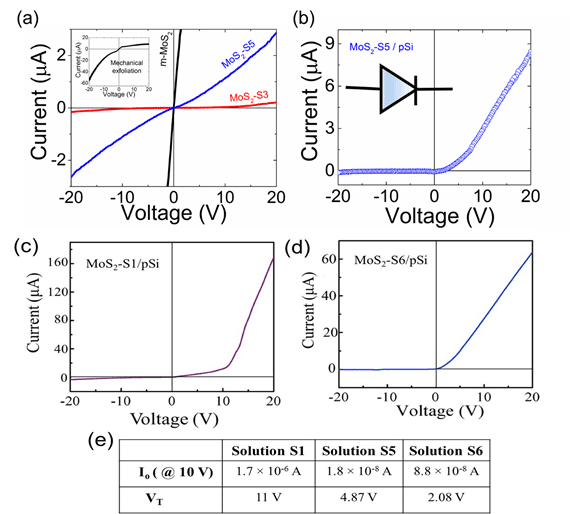
(a) I-V characteristic of mechanically exfoliated m-MoS2 and drop cast MoS2 dispersed in conditions of solution chemistry S3 with Mo electrodes (red) and MoS2 dispersed in conditions of S5 (blue) deposited on SiO2/Si. (b) I-V characteristic of p-n junction formed with MoS2-S5 on doped p-Si substrate showing current rectification in the reverse bias regime with VT ~ 4.87 V. Inset shows symbol for two-terminal current rectifying diode. In (c) and (d), the I-V data for solution chemistry S1 and S6 is also shown where the inks were dispersed onto heavily doped p-type Si substrates, similar to the data obtained in (b). We see the effect of solution chemistry on the device parameters, specifically leakage current Io and turn on voltage, VT. The device figures of merit summarized in (e) indicate that the solution chemistry S6 yields the lowest VT of ~ 2 V, as compared to S5 which has a turn on voltage of ~ 4.87 V and S1 with VT ~ 11 V; the leakage currents for both of the Terpineol derived solution chemistries, S5 and S6 yield Io ~ on the order of 10 – 90 nA, whereas the Io for S1 is ~ 2 mA, which is approximately two orders of magnitude larger. All of this data once again validates the effectiveness of Terpineol to enable 2D dispersions that yield enhanced transport properties, which is also consistent with the enhanced excitonic effects observed in this particular solution chemistry.
Environmentally Friendly Inks
Our group is also looking at environmentally friendly formulations of inks. In our recent work, a surfactant–free graphene ink was prepared in a mixture of Terpineol (T) and Cyclohexanone (C) and optimized to yield rheologies appropriate for ink-jet printing on both rigid SiO2/Si and flexible polyimide substrates. The surfactant-free ink optimized here, were used to demonstrate enhanced electrical transport characteristics for graphene, where resistivity values are significantly lower compared to surfactant-assisted formulations derived from N-methyl-2-pyrrolidone (NMP) and Ethyl Cellulose (EC). The C:T surfactant-free ink is stable with aging, exhibiting minimal signs of graphene nano-membrane re-agglomeration.
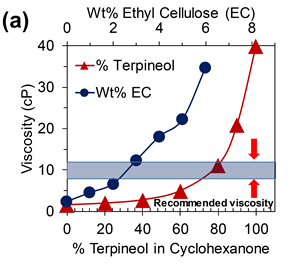
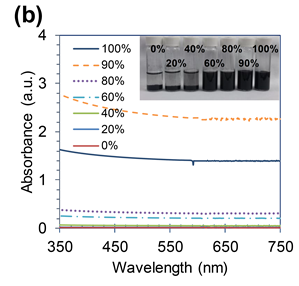
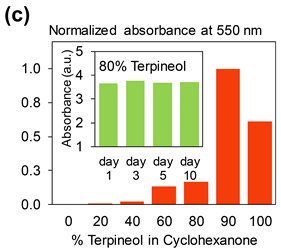
The other dispersions of interest for our group involve looking at zero-dimensional fullerenes, black phosphorus, perovskites, WSe2 and h-BN.
The Cary UV-VIS-NIR spectrophotometer that we commonly use in our research to perform Optical Absorption Spectroscopy